
Precision Oncology
White Paper
ABSTRACT
The landscape of oncology care is rapidly evolving through digital innovation, as part of a broader healthcare transformation where digital health venture funding reached $10.1B last year. In the cancer space specifically, the Division of Cancer Control and Population Sciences invested nearly $583 million in 2024 across approximately 925 grants aimed at improving cancer outcomes and patient experiences.
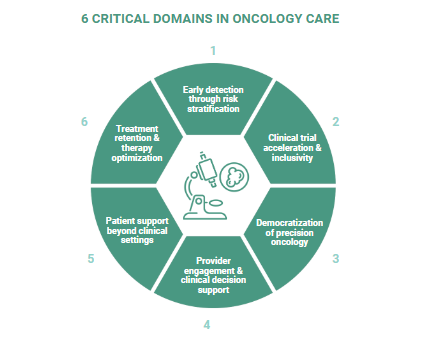
This white paper explores how digital transformation is revolutionizing cancer care across six critical domains: early detection through risk stratification, clinical trial acceleration, democratization of precision oncology, provider engagement, patient support beyond clinical settings, and treatment optimization. Despite remarkable advances in cancer therapeutics, significant gaps persist in diagnosis, access, and treatment efficacy. Digital approaches offer practical solutions to these challenges, creating opportunities for stakeholders throughout the oncology ecosystem to collaborate more effectively. This paper outlines how digital transformation is reshaping oncology across six critical domains—reframing persistent challenges and revealing strategic opportunities for pharmaceutical companies to play a more integrated role in delivering high-impact cancer care.
INTRODUCTION: THE DIGITAL IMPERATIVE IN ONCOLOGY
The practice of oncology stands at a pivotal crossroads. On one side lies unprecedented scientific progress with breakthrough targeted therapies, immunotherapies, and our expanding understanding of cancer biology. On the other side, stubborn gaps in implementation, including delayed diagnoses, limited access to advanced treatments, suboptimal therapy adherence, and care fragmentation. These gaps represent not just missed opportunities for better patient outcomes but also significant inefficiencies in our healthcare systems and markets.
Digital transformation offers a bridge between oncology's scientific potential and its practical reality. Through thoughtfully designed digital approaches, stakeholders across the oncology ecosystem can address longstanding challenges in ways that were previously impossible. Digital innovation is creating a meaningful impact across six critical domains in oncology care, presenting strategic implications for pharmaceutical companies seeking to optimize both patient care and market performance.
PART 1: EARLY DETECTION THROUGH RISK STRATIFICATION
The promise of early cancer detection remains one of medicine's most compelling opportunities.
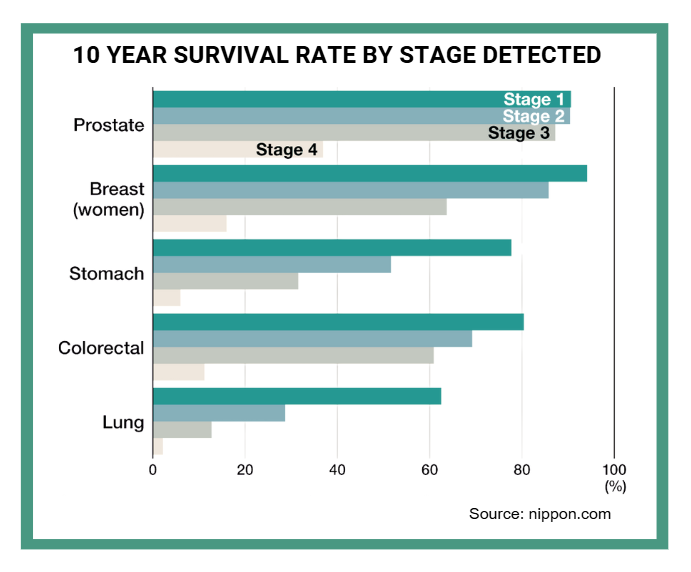
The survival statistics tell a stark story: Stage I colorectal cancer offers a 92% five-year survival rate, while Stage IV plummets to just 15.6%. Similar patterns exist across most cancer types, creating both moral and economic imperatives to move diagnosis earlier in disease progression.
What's particularly striking about the current approach is how cancer screening is structured around broad population demographics rather than individualized risk profiles. For example, women are screened for breast cancer at age 40 or 50 based on general guidelines, but rarely is the constellation of genetic, environmental, and lifestyle factors that truly determine risk incorporated.
This approach is changing rapidly through digital innovation in risk stratification. At recent CancerX Executive Roundtables, health system leaders have consistently highlighted early detection as a priority. These leaders specifically emphasize "high-risk surveillance" as a critical initiative, describing their focus as identifying patients at high risk of cancer and getting them into appropriate screening pathways within their catchment areas. Oncology leaders at large health systems underscore the strategic importance of integrating cancer screening throughout primary care networks and the broader healthcare ecosystem.

These leaders recognize that traditional broad population screening approaches are giving way to more sophisticated risk stratification methods. Digital tools now enable the identification of high-risk individuals who would benefit from earlier or more frequent screening based on multiple risk factors beyond simple demographics.
STRATEGIC IMPLICATIONS FOR PHARMACEUTICAL COMPANIES
For pharmaceutical companies developing cancer therapies, these developments create profound strategic implications. First, they signal a market shift toward earlier-stage treatment paradigms, potentially expanding patient populations for therapies historically used in later-stage disease. Second, they introduce opportunities for pharma to engage patients much earlier in their journey, transforming market development activities and patient education approaches.
The most forward-thinking pharma companies are already positioning themselves at this intersection of early diagnosis and treatment. Rather than viewing biomarker testing as something that happens after diagnosis, they're partnering with diagnostic innovators to create integrated pathways that connect risk identification directly to appropriate testing and subsequent treatment options.
However, this shift requires pharmaceutical companies to think differently about patient journeys. What if your therapy's patient journey doesn't begin at diagnosis but rather at the point of initial risk identification? What if market development activities should target not just oncologists but also primary care physicians and genetic counselors who participate in these emerging risk stratification programs?
The healthcare systems implementing these approaches are achieving what once seemed impossible: identifying cancers at significantly earlier stages while simultaneously reducing overall screening costs through strategic resource allocation. For pharma companies developing oncology portfolios, this represents both an opportunity and a strategic imperative to rethink how patients are identified, engaged, and ultimately supported through their treatment journey.
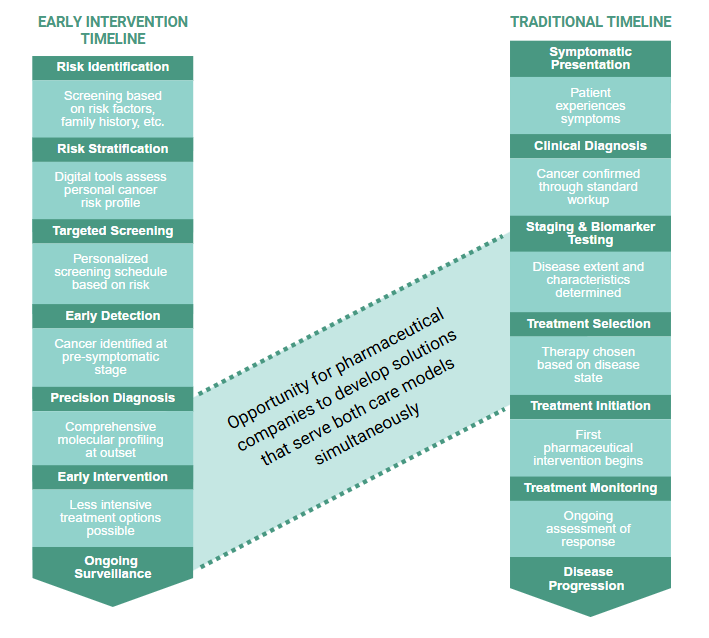
REIMAGINED PATIENT JOURNEY
The evolution from reactive to proactive cancer care is fundamentally reshaping patient journeys and expanding opportunities for pharmaceutical engagement. The traditional pathway, beginning at symptomatic presentation and clinical diagnosis, contrasts sharply with the emerging early intervention model that initiates at risk identification and stratification. While these journeys appear distinct, they intersect critically at precision diagnosis and biomarker testing—a convergence point where comprehensive molecular profiling guides treatment decisions in both models. This intersection represents a strategic opportunity for pharmaceutical companies to bridge traditional and emerging care paradigms, creating integrated solutions that serve patients regardless of where they enter the cancer care continuum.
PART 2: CLINICAL TRIAL ACCELERATION & INCLUSIVITY
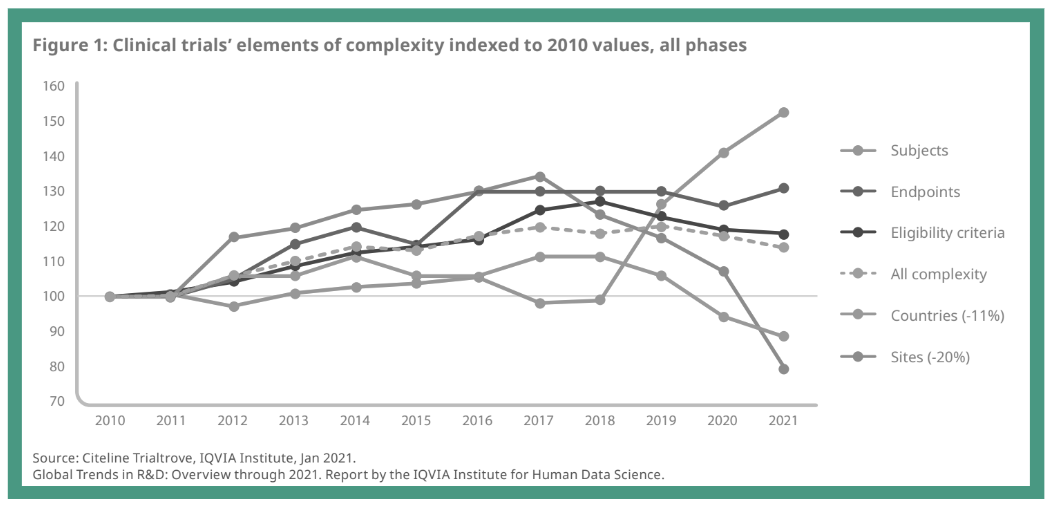
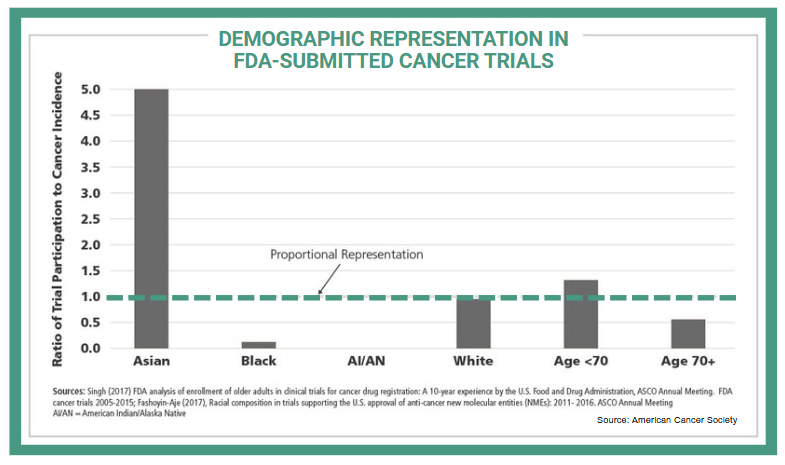
Clinical trials in oncology are becoming increasingly complex, creating significant challenges in patient recruitment, retention, and trial completion. The fundamental mismatch between where most cancer patients receive care and where most clinical trials are conducted represents one of oncology's most persistent challenges. Only 7.1% of adult cancer patients participate in trials, and those who do participate rarely reflect the diversity of the broader patient population. Recent analyses of cancer therapeutic trials found that only 4%-6% of trial participants are Black and 3%-6% are Hispanic, despite representing 15% and 13% of people with cancer, respectively.
DIGITAL SOLUTIONS FOR CLINICAL TRIAL ACCELERATION
Digital approaches are beginning to bridge this gap with practical, implementable solutions. Site-agnostic screening tools that analyze EHR data across healthcare networks can identify eligible patients regardless of where they receive care. These platforms automatically match patient profiles against trial criteria, flagging potential candidates for coordinators to review. Organizations implementing these tools have reduced screening time by 80% while significantly diversifying their participant pools.
Decentralized trial components enable participation without requiring frequent visits to academic centers. AstraZeneca demonstrates this impact: their implementation of digital tools in a COPD trial is predicted to reduce treatment duration and in-person visits by 50%, decrease overall trial duration by 15%, cut costs by 32%, and improve the patient experience index by 68%. This isn't just convenient—it fundamentally changes who can participate by removing geographic barriers.
A study indicated that 80% of sites using telehealth visits found them effective, and 85% reported that remote collection patient-reported symptoms was beneficial; additionally, 89% felt that remote consenting was effective. These approaches address practical problems rather than hypothetical ones.

STRATEGIC IMPLICATIONS FOR PHARMACEUTICAL COMPANIES
For pharmaceutical companies, the benefits extend beyond speed to market. Trials that reflect actual patient populations generate more applicable safety and efficacy data, potentially reducing post-approval surprises and supporting stronger market uptake. Additionally, positive participant experiences create meaningful goodwill within patient communities.
The most effective acceleration strategies don't simply digitize existing processes—they fundamentally rethink who can participate and how. This perspective shift represents the true promise of digital innovation in clinical trials, with the potential to deliver therapies to market faster while generating more representative and applicable data that could reduce post-approval surprises and support stronger market uptake.
PART 3: DEMOCRATIZING PRECISION ONCOLOGY
While breakthrough targeted therapies continue to emerge at unprecedented rates, implementation lags significantly behind innovation. The most advanced treatments, from targeted therapies to immunotherapies to cell-based approaches, remain concentrated in academic medical centers and specialized cancer institutes, creating a significant geographic disparity in care access.
This growing divide between scientific advancement and clinical implementation represents both a market inefficiency and a missed opportunity for patient impact. As one health system leader at a CancerX Roundtable pointedly observed, "Most patients aren't at [renowned institutions]—they're in community hospitals with fewer resources." This geographic disparity creates a "postal code lottery" where precision medicine access depends largely on proximity to academic centers.

The barriers to precision medicine adoption in community settings extend far beyond simple availability. Community hospitals and practices face complex implementation challenges including provider expertise limitations, workflow integration hurdles, reimbursement navigation complexities, and infrastructure constraints. For many patients living in rural or underserved areas, accessing cutting-edge therapies would require traveling hundreds of miles—a burden that proves prohibitive for many, especially those most vulnerable.
EXPANDING ACCESS THROUGH DIGITAL TOOLS
Digital approaches offer practical solutions to narrow this divide. Clinical decision support tools integrated at the point of care can significantly increase appropriate testing and treatment rates. By embedding evidence-based recommendations within EHR workflows, these systems remind oncologists when patients should receive testing and suggest targeted treatment options based on molecular findings.
Virtual tumor boards enable expertise sharing without requiring physical proximity. When community oncologists can easily connect with subspecialists to review complex cases, appropriate therapy utilization improves substantially. This approach recognizes the reality that keeping pace with the rapidly evolving precision oncology landscape exceeds any individual community oncologist's capacity, given their broader practice requirements.
The persistent gap between innovation concentration and widespread implementation represents both a clinical tragedy for individual patients who might benefit from precision approaches and a significant market inefficiency that undermines the return on substantial R&D investments in advanced oncology therapeutics. By embracing digital democratization strategies, pharmaceutical companies can expand their therapeutic reach while transforming patient outcomes across diverse practice settings—truly delivering on the promise of precision medicine for all patients, not just those with geographic proximity to specialized centers.
PARTNERSHIP OPPORTUNITIES IN PRECISION ONCOLOGY
For pharmaceutical companies developing precision therapies, these solutions create natural partnership opportunities. Rather than simply launching targeted therapies and hoping for the best, forward-thinking companies are developing comprehensive digital ecosystems around their products. These approaches don't just drive utilization—they ensure that appropriate patients receive effective treatments.
Companies can pursue multiple partnership routes to enhance clinical trial access and therapy delivery in community settings. Implementing hub-and-spoke models allows pharmaceutical companies to centrally organize research coordination and data management at academic centers while extending trial access to community hospitals. This approach unburdens community settings from complex administrative tasks while freeing up capacity for research coordinators. By strategically investing in digital solutions that support these models, pharmaceutical partners can simultaneously democratize access to cutting-edge treatments and address the persistent challenge of finding enough eligible patients for increasingly targeted therapies.

PART 4: PROVIDER ENGAGEMENT & CLINICAL DECISION SUPPORT
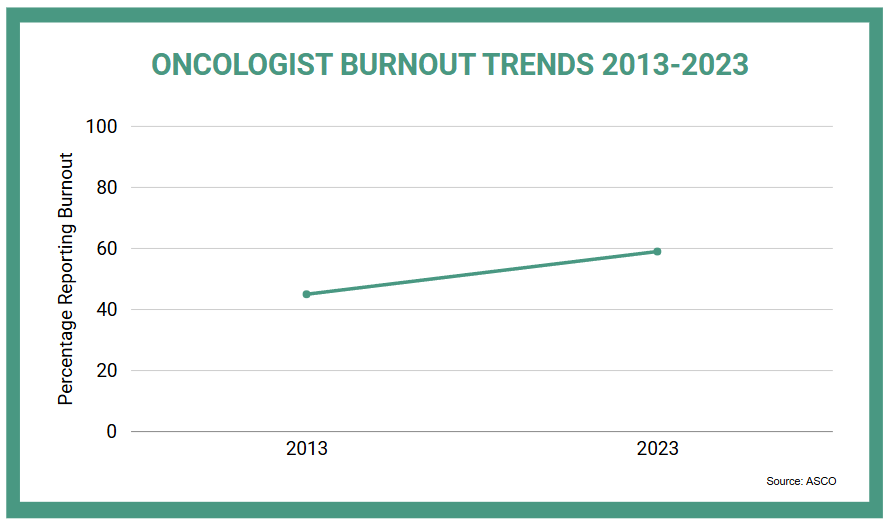
The traditional provider engagement model is increasingly misaligned with clinical realities. Oncologists face mounting time pressure, information overload, and restricted access policies. Among 328 oncologists who responded to a 2023 questionnaire about professional well-being and satisfaction with their jobs, the burnout rate was 59% compared with 45% in a similar survey from 2013. This requires a fundamental rethinking of how scientific exchange occurs.
In terms of the length of time physicians have been experiencing information overload,
39.6% claimed that the phenomenon had increased in the last 5-6 years; 10.9% confirmed that it started before that, about 10 to 15 years ago; and 14.9% did not specify a date but listed aspects such as progressing in their career, noting how the pressure shifted from night shifts only to an all-time problem and overload worsening once email was firmly established within healthcare systems.
Participants at a CancerX Executive Roundtable highlighted this growing crisis, with one oncology leader noting that "administrative burden" now ranks among their top three operational challenges. Another participant observed that "oncologists face mounting time pressure and information overload," creating a situation where "the specialized nature of oncology makes keeping current with rapidly evolving evidence increasingly difficult."
Traditional provider engagement approaches often compound these challenges rather than alleviating them. As one oncologist candidly shared, "I don't have time for another educational program, but I desperately need tools that reduce my administrative burden and help me make better decisions more efficiently."
FOCUS PROVIDER ENGAGEMENT ON UNBURDENING & VALUE CREATION
This shift in expectations is driving a new standard: provider engagement must actively unburden. Digital approaches can transform this dynamic when designed around provider needs, and the most effective tools don’t just deliver information; they simplify decision-making, remove workflow friction, and free up clinical capacity.
Consider AI-enabled protocol standardization platforms. These systems reduce the time required to verify complex oncology treatment plans, ensuring compliance while minimizing manual input. The solutions provide context-aware recommendations that ensure adherence to complex oncology protocols with limited manual verification needed. As a result, the time savings on protocol verification allow nurses to free up time for more valuable activities and sometimes even allows health systems to establish dedicated new clinics that become a new revenue center for the clinic. Similarly, virtual tumor board platforms are reshaping complex case management. By automatically aggregating relevant patient data into a single view, these tools dramatically reduce case preparation time, allowing more patients to benefit from subspecialist input without delaying care.
What unites these examples is not the technology itself, but the intent: to return time, clarity, and capacity to clinicians. Success is measured not by engagement metrics but by time saved, processes streamlined, and new capabilities enabled.
For pharmaceutical organizations, this evolution offers a new way to engage by solving real-world workflow challenges rather than sharing scientific messages. A value-centered approach begins by asking: What are the daily pain points for oncologists? Where does friction limit care quality or efficiency? How can we design tools and collaborations that eliminate those constraints?
Reframing medical engagement around unburdening rather than educating unlocks a more trusted and impactful role. It positions digital solutions as essential clinical enablers. And it aligns industry activity with the most pressing priorities of modern oncology care: improving quality, preserving clinician well-being, and making it possible to do more with the same resources.
In a landscape where every minute counts, digital strategies must earn their place by giving time back.
Part 5: PATIENT SUPPORT BEYOND CLINICAL SETTINGS
Cancer care extends far beyond the clinic walls, creating a critical need for support systems that reach patients where they actually spend most of their time—in their homes and communities.
The stark reality of cancer care is that patients spend very little time in clinical settings, yet must manage their condition 24/7. In one analysis, it was reported that patients with metastatic breast cancer spent approximately 7% to 10% of their days engaged in healthcare activities during the initial months of treatment. This includes time spent traveling to appointments and receiving care, indicating that while they are physically present in clinical settings for a fraction of their time, the overall burden of managing their cancer care is substantial.
Research on adolescents and young adults (AYA) with cancer shows that 56-75% of survivors who needed specific supportive care services, such as support groups, pain management, or mental health services, did not receive them. This reveals significant gaps in continuous care and support.

CONTINUOUS PATIENT SUPPORT MODELS
Digital approaches enable a more continuous, accessible support model. Remote symptom monitoring platforms allow patients to report symptoms between visits, creating visibility into previously unseen aspects of the cancer experience. These tools don't just collect data—they trigger appropriate interventions when needed. An algorithm-based intervention in a community oncology network significantly increased completed palliative care visits from 8.3% to 43.9% among high-risk patients while decreasing end-of-life systemic therapy from 16.1% to 6.5%. This demonstrates the effectiveness of algorithm-driven triage in improving patient care outcomes by addressing needs before they escalate.
Virtual navigation expands reach while maintaining the human connection critical to cancer care. This approach recognizes that technology alone isn't the answer—people need both information and empathetic support throughout their cancer journey. Virtual navigation programs in oncology have been shown to significantly increase patient access, with 58% of users being from outside the local area, compared to only 42% local users.
IMPROVED OUTCOMES THROUGH DIGITAL SUPPORT
For pharmaceutical companies, these platforms create unprecedented opportunities to understand the complete patient experience with their therapies. One study demonstrated that digital symptom monitoring systems, which allow patients to report symptoms weekly, can improve symptom control and overall quality of life. These systems enable healthcare providers to identify and address issues early, thereby reducing the likelihood of treatment discontinuation due to manageable side effects like nausea or fatigue.
Texas Oncology partnered with Canopy for remote patient monitoring, where Canopy reported it has seen a 45% improvement in treatment persistence after three months as well as a 22% reduction in ER visits and hospitalizations per 100 patient months, along with 88% sustained patient engagement at six months. It has also led to a 37% reduction in treatment discontinuation at three months and improved the early detection of toxicities with bispecific antibody therapies, according to the company.
These models are especially critical in the context of advanced therapies like CAR-T, where the clinical and logistical burden of administration—such as hospitalization requirements and intensive post-treatment monitoring—can limit access and uptake. Digital innovations that support safer outpatient administration, enable home-based monitoring, and proactively manage complications can help overcome these access barriers. In doing so, they not only expand the eligible patient population but also enhance therapy delivery in real-world settings.
What makes these approaches successful isn't simply their digital delivery but rather their ability to address unmet needs that traditional models cannot. By extending support beyond clinic walls, we can fundamentally transform the cancer experience while ensuring therapies deliver their full potential benefit.
Part 6: TREATMENT RETENTION- OPTIMIZING THERAPY DURATION AND EFFECTIVENESS
The gap between clinical trial efficacy and real-world effectiveness often stems from suboptimal treatment persistence.
A study indicated that nearly 20% of patients with stomach cancer, about 30% with renal cancer, and nearly 50% with liver cancer discontinued their oral anti-cancer medications (OAMs), reflecting a range of discontinuation rates across different cancer types. This represents both a patient care problem and a significant market challenge for innovative therapies.
Improving persistence is not simply a matter of encouraging patients to “stay on therapy.” It requires addressing the root causes that drive early drop-off: unmanaged side effects, financial strain, lack of clarity about treatment benefit, and low confidence in self-management. These challenges evolve over the course of the patient journey and require dynamic solutions—not static interventions.
Digital technologies are well-positioned to address this complexity. The most effective approaches go beyond generic adherence reminders to offer real-time insight into patient status, structured triage of emerging symptoms, and adaptive support tailored to where a patient is in their treatment arc. These tools can flag signs of risk early—before discontinuation becomes likely—and enable timely, personalized intervention.
A shift is underway: from viewing digital tools as supplementary add-ons to recognizing them as integral to therapeutic strategies. The goal is not just to digitize existing workflows, but to reimagine how patients are supported between visits—when most decisions about whether to continue treatment are actually made.
Leading organizations are starting to build very robust retention strategies to enhance treatment outcomes. These strategies integrate digital tools for side effect triage, behavioral nudges, patient education, and benefit navigation—together forming a coordinated response to the known points of friction that drive drop-off.
Success in this domain requires reframing persistence as a system design challenge. When digital solutions are aligned to patient needs, tailored over time, and embedded into care pathways, treatment persistence improves—and with it, the full value of innovation is more likely to be realized.
FUTURE DIRECTIONS
As oncology evolves toward greater molecular precision, long-standing distinctions between cancer and rare disease are becoming less relevant. Many of today's cancer therapies are developed for molecularly defined subpopulations that affect a fraction of patients across many tumor types, such as tumors with NTRK fusions, EGFR exon 20 insertions, or BRAF mutations. These are rare diseases in every operational sense: hard to find, harder to diagnose, and difficult to serve through traditional infrastructure.
This evolution brings with it a new strategic imperative: precision medicine demands precision infrastructure. Consider biomarker testing. In both precision oncology and rare conditions, testing is often available but inconsistently applied. In one meta-analysis, nearly 60% of patients who received comprehensive genomic profiling had actionable alterations—yet only 15% went on to receive targeted treatment. The diagnostic odyssey seen in rare disease is mirrored here: long delays, fragmented pathways, and underutilized insights.
Pharmaceutical companies are often running separate strategies for oncology and rare disease, but that division no longer reflects how care is delivered. Both areas now rely on solving the same set of challenges: finding the right patients, ensuring timely and equitable testing, navigating reimbursement, and coordinating care across specialties. The tools required—like digital patient identification, integrated diagnostics, and cross-specialty pathways—are increasingly the same in both cases.
For pharmaceutical organizations, this convergence presents both an operational opportunity and a structural challenge. To lead in this evolving landscape, forward-thinking companies adapt their approaches in several key areas:
Break down internal silos: Rethink the traditional separation between oncology and rare disease functions. Align teams and resources around shared priorities such as patient identification, diagnostic enablement, and stakeholder education—areas that increasingly transcend therapeutic boundaries.
Adopt diagnostic-first engagement models: As molecular markers become the primary determinant of treatment eligibility, engagement strategies must shift accordingly. Rather than centering around tumor type, outreach efforts should begin at the point of biomarker and genomic testing, where the patient journey often truly begins.
Engage the broader ecosystem: Success in reaching small, dispersed patient populations depends on collaboration. Strategic partnerships with diagnostic innovators, digital health platforms, advocacy organizations, and care networks are essential to build the infrastructure and trust required for effective intervention.
WHAT MIGHT THIS APPROACH LOOK LIKE IN PRACTICE?
Cross-disciplinary medical education can connect oncologists with geneticists and rare disease specialists to share diagnostic approaches and referral pathways. Integrated diagnostic partnerships leverage common infrastructure to support both cancer biomarker testing and rare disease genetic testing, potentially reducing implementation barriers for healthcare systems. Collaborative patient identification initiatives combine digital technologies, provider education, and awareness campaigns to recognize both rare cancers and traditional rare diseases earlier in their progression. Shared policy advocacy addresses common barriers like insurance coverage for advanced diagnostics, recognition of centers of excellence, and accelerated regulatory pathways for targeted treatments.
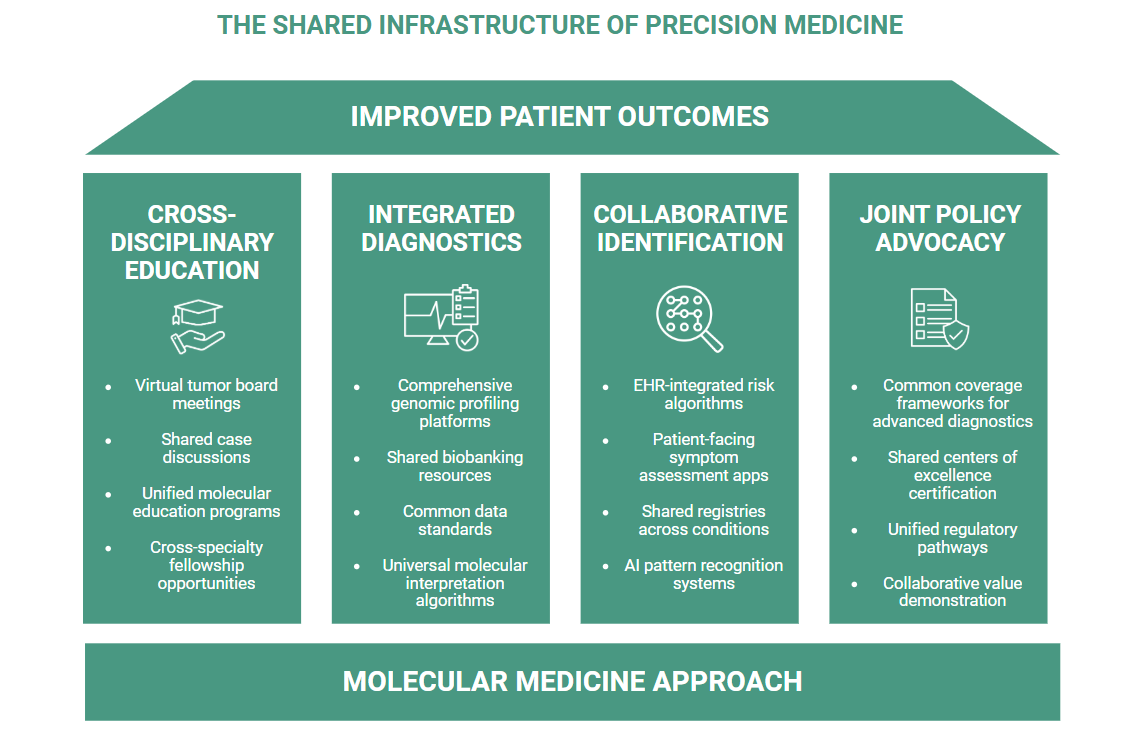
These ecosystem approaches reflect a fundamental reality: in an era of precision medicine, the boundaries between disease categories matter less than the shared challenges of identifying and treating molecularly-defined patient populations.
The pharmaceutical companies that embrace this convergence gain significant advantages beyond efficiency. By engaging ecosystem partners across traditional boundaries, they position themselves as forward-thinking collaborators focused on patient needs rather than legacy categorizations. This approach builds deeper relationships with key stakeholders while addressing the fundamental challenges that ultimately determine whether innovative therapies reach the patients who need them.
The future belongs to organizations willing to transcend traditional categorization in pursuit of more effective ecosystem collaboration. The question isn't whether your company works in oncology or rare disease—it's whether you're building integrated partnership approaches that reflect the biological and clinical reality patients experience every day.
CONCLUSION
The digital transformation of oncology represents far more than a series of technological innovations. It offers a fundamental reimagining of how cancer care is delivered, experienced, and optimized. As this white paper has demonstrated, digital approaches are creating a measurable impact across the entire cancer care continuum—from risk-stratified early detection to precision diagnosis, from inclusive clinical trials to democratized treatment access, from meaningful provider engagement to continuous patient support and optimized treatment persistence.
The evidence presented throughout this paper points to a clear conclusion: the most successful digital strategies aren't those that simply digitize existing processes but rather those that fundamentally rethink care models to address longstanding gaps that traditional approaches cannot bridge. For pharmaceutical companies, this represents both an opportunity and an imperative to evolve beyond traditional drug development to become ecosystem partners in improving the entire cancer care journey.
The implications for strategic planning are significant. Partnership strategies must expand beyond conventional boundaries to include diagnostic innovators, digital health companies, health systems implementing new care models, and patient communities demanding better experiences. Digital health portfolio strategies must move from fragmented pilots to coherent ecosystems that address multiple interconnected challenges simultaneously.
As the convergence of oncology with rare disease illustrates, the future belongs to organizations willing to transcend historical categorizations in pursuit of more effective ecosystem collaboration. By embracing this collaborative mindset and leveraging the digital approaches described in this paper, stakeholders across the oncology ecosystem can work together to ensure that scientific progress translates into meaningful improvements in patient outcomes, system efficiency, and market performance.
The path forward is clear—digital transformation offers unprecedented opportunities to bridge longstanding gaps in cancer care. The only remaining question is which organizations will lead this transformation and which will be left behind.
Accelerate your healthcare innovation today.
Contact Decimal Health to drive decisive results and conquer market challenges with precision.
SOURCES
- American Cancer Society. (2018, May 11). Figure 6. demographic representation in FDA-submitted Cancer trials. American Cancer Society Cancer Action Network. https://www.fightcancer.org/figure-6-demographic-representation-fda-submitted-cancer-trials
- Ami Vyas, PhD, MS, MBA AVyas@URI.edu, Andrew Descoteaux, MS, Stephen Kogut, PhD, MBA, RPh, Megha A Parikh, PhD, MS, Patrick J Campbell, PhD, PharmD, RPh, Amanda Green, MPH, and Kimberly Westrich, MA. (2022, July 25). Predictors of adherence to oral anticancer medications: An analysis of 2010-2018 US nationwide claims | Journal of Managed Care & Specialty Pharmacy. JMCP. https://www.jmcp.org/doi/10.18553/jmcp.2022.28.8.831
- AstraZeneca. (2024, April 5). Our oncology R&D strategy. Our Oncology R&D strategy. https://www.astrazeneca.com/media-centre/articles/2024/oncology-r-and-d-strategy-driving-innovation.html
- Bai, N. (2023, June 5). Few patients receive recommended genetic testing after cancer diagnosis. News Center. https://med.stanford.edu/news/all-news/2023/06/genetic-testing-cancer.html
- Cavallo, J. (2021, November 17). Digital symptom monitoring system may improve patients’ symptom control and physical function during cancer therapy. The ASCO Post. https://ascopost.com/news/november-2021/digital-symptom-monitoring-system-may-improve-patients-symptom-control-and-physical-function-during-cancer-therapy/
- Duran, C., & Bonham, M. (2023, November). Clinical innovation: How digital health solutions are transforming our trials. AstraZeneca. https://www.astrazeneca.com/what-science-can-do/topics/clinical-innovation/clinical-innovation-digital-health-solutions-transforming-trials.html
- Gliadkovskaya, A. (2025, March 18). Texas oncology partners with canopy on Remote Patient Monitoring. Fierce Healthcare. https://www.fiercehealthcare.com/health-tech/texas-oncology-partners-canopy-remote-monitoring
- Ioannis Zerdes, MD, PhD, Panagiotis Filis, MD, Georgios Fountoukidis, MD, Ali Inan El-Naggar, MD, Foteini Kalofonou, MD, PhD, Antonio D’Alessio, MD, Athanasios Pouptsis, MD, Theodoros Foukakis, MD, PhD, George Pentheroudakis, MD, PhD, Johan Ahlgren, MD, P. (2025, January 22). Comprehensive genome profiling for treatment decisions in patients with metastatic tumors: real-world evidence meta-analysis and registry data implementation. Oxford Academic. https://academic.oup.com/jnci/advance-article/doi/10.1093/jnci/djab044/6178006
- J;, H. S. (2024). Impact of virtual navigation on the education and access of patients with cancer: A national mixed methods investigation. SAGE open nursing. https://pubmed.ncbi.nlm.nih.gov/39380928/
- Julien Poulain . (2024, June 10). Major survey reveals lengthy diagnostic delays for rare disease patients . EURORDIS. https://www.eurordis.org/survey-reveals-lengthy-diagnostic-delays/
- Landi, H. (2025, January 13). Digital Health Venture funding hit $10.1B in 2024 as investors focused on earlier-stage dealmaking. Fierce Healthcare. https://www.fiercehealthcare.com/digital-health/digital-health-venture-funding-hit-101b-2024-investors-focused-earlier-stage-deals
- Lee, K., Kim, S., Kim, S. H., Yoo, S.-H., Sung, J. H., Oh, E. G., Kim, N., & Lee, J. (2023, January 6). Digital Health Interventions for adult patients with cancer evaluated in randomized controlled trials: Scoping review. Journal of medical Internet research. https://pmc.ncbi.nlm.nih.gov/articles/PMC9862347/
- MADELUNG, M. (2022). Finding All the Needles in the Haystack: Technology-Enabled Patient Identification for Clinical Trials. Frankfurt; IQVIA. Electronically accessed: https://www.iqvia.com/-/media/iqvia/pdfs/library/white-papers/finding-all-the-needles-in-the-haystack-technology-enabled-patient-identification.pdf
- Markman, M. (2022, August 11). Colorectal cancer stages and survival rate. City of Hope. https://www.cancercenter.com/cancer-types/colorectal-cancer/stages
- NCI Division of Cancer Control and Population Sciences. (n.d.). 2024 overview and highlights. DCCPS 2024 Overview and Highlights. https://cancercontrol.cancer.gov/overview-highlights/2024/investments_in_cancer_control.html
- Nippon. (2023, July 7). Early detection crucial: Japan’s 10-year cancer survival rate stands at 53.3%. nippon.com. https://www.nippon.com/en/japan-data/h01626/early-detection-crucial-japan%E2%80%99s-10-year-cancer-survival-rate-stands-at-53-3.html
- Osterwail, N. (2025, February 4). Most oncologists are dissatisfied and burned out, asco ... Oncology News Central. https://www.oncologynewscentral.com/oncology/most-oncologists-are-dissatisfied-and-burned-out-asco-report-shows
- Randall A. Oyer, MD Patricia Hurley, MSc Leigh Boehmer, PharmD Suanna Steeby Bruinooge, MPH Kathryn Levit, PhD Nadine Barrett, PhD Et al. (2022, May 19). Increasing racial and ethnic diversity in cancer clinical trials: An American Society of Clinical Oncology and Association of Community Cancer Centers Joint Research statement | journal of clinical oncology. ASCO Publications. https://ascopubs.org/doi/10.1200/JCO.22.00754
- Ravi B. Parikh, MD, MPP, et al. (2025, February 21). Algorithm-based palliative care in patients with cancer: A cluster randomized clinical trial | oncology | jama network open | jama network. JAMA Network. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2830599
- Rawla, P., Sunkara, T., & Barsouk, A. (2019). Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Przeglad gastroenterologiczny. https://pmc.ncbi.nlm.nih.gov/articles/PMC6791134/
- Rocque GB;Williams CP;Ingram SA;Azuero A;Mennemeyer ST;Young Pierce J;Nipp RD;Reeder-Hayes KE;Kenzik KM; (2020, November 9). Health care-related time costs in patients with metastatic breast cancer. Cancer medicine. https://pubmed.ncbi.nlm.nih.gov/32955793/
- Rocque, G. B., Williams, C. P., Ingram, S. A., Azuero, A., Mennemeyer, S. T., Young Pierce, J., Nipp, R. D., Reeder-Hayes, K. E., & Kenzik, K. M. (2020, November). Health care-related time costs in patients with metastatic breast cancer. Cancer medicine. https://pmc.ncbi.nlm.nih.gov/articles/PMC7666754/
- Sbaffi, L., Walton, J., Blenkinsopp, J., & Walton, G. (2020, July 27). Information overload in emergency medicine physicians: A multisite case study exploring the causes, impact, and solutions in four North England National Health Service Trusts. Journal of medical Internet research. https://pmc.ncbi.nlm.nih.gov/articles/PMC7418008/#abstract1
- Smeltzer, M. P., Scagliotti, G. V., Wakelee, H. A., Mitsudomi, T., Roy, U. B., Clark, R. C., Arndt, R., Pruett, C. D., Kelly, K. L., Ujhazy, P., Johnson, M. L., Eralp, Y., Barrios, C. H., Barlesi, F., Hirsch, F. R., & Bunn, P. A. (2022, May). International Association for the study of lung cancer study of the impact of coronavirus disease 2019 on International Lung Cancer Clinical Trials. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. https://pmc.ncbi.nlm.nih.gov/articles/PMC8851565/
- Smith, A. W., Parsons, H. M., Kent, E. E., Bellizzi, K. M., Zebrack, B. J., Keel, G., Lynch, C., Rubenstein, M. B., & Keegan, T. H. M. (2013, March 23). Unmet support service needs and health-related quality of life among adolescents and young adults with cancer: The aya hope study. Frontiers. https://www.frontiersin.org/journals/oncology/articles/10.3389/fonc.2013.00075/full
- Unger JM;Shulman LN;Facktor MA;Nelson H;Fleury ME; (2024, June 20). National estimates of the participation of patients with cancer in clinical research studies based on commission on Cancer Accreditation Data. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. https://pubmed.ncbi.nlm.nih.gov/38564681/
- Westphalen, C. B., Krebs, M. G., Le Tourneau, C., Sokol, E. S., Maund, S. L., Wilson, T. R., Jin, D. X., Newberg, J. Y., Fabrizio, D., Veronese, L., Thomas, M., & de Braud, F. (2021, July 20). Genomic context of NTRK1/2/3 fusion-positive tumours from a large real-world population. Nature News. https://www.nature.com/articles/s41698-021-00206-y
.png?width=125&height=125&name=logo_wt%20(3).png)




.png?width=300&name=CancerX%20Decimal%20Banner%20(2).png)